Recap: ITCS 2022
For a long time, it was believed that a PhD meant a career path towards becoming a tenure-track professor. However, the reality is that only about 20% of those who earn a PhD in Canada end up in tenure-track positions after completion (UofT 10,000 PhDs project). Universities offer trainees limited opportunities to gain experience in alternative career paths, leaving many confused on what to pursue post-graduation. According to UofT’s 10,000 PhDs project, about 40% of life sciences PhD trainees transition to exciting careers in industry, and that number is growing along with a boom in the Canadian biotech and pharma landscapes (UofT 10,000 PhDs project). As part of LSCDS’s mission to bridge the gap between academia and industry, the Industry Team Case Study (ITCS) was created to provide trainees with hands-on experience in industry while completing their degrees. Since its launch in 2016, the ITCS has provided 180 trainees with experience in developing industry projects under the guidance of industry professionals from companies like Moderna, Roche, and Astrazeneca.
The ITCS is a four-month long engagement in which trainees work together in teams of 3-4 members to complete a team-defined project that simulates what working in the pharmaceutical industry would look like. Each team has a panel of industry advisors that provides them with monthly insight and guidance in developing their project. At the Showcase and Networking event, trainees present their projects to advisors and their ITCS peers in 10-minute presentations, followed by a networking session. The event provides an added opportunity to meet advisors from other sectors and gain industry network connections. Ending the year on a high note, this was LSCDS’s last event of the academic year and first in-person event in two years!
This year, the ITCS projects fell under three main sectors: medical affairs, regulatory affairs, and business and market access. I had the pleasure of attending several presentations from the medical affairs and regulatory affairs sectors. One medical affairs presentation described how their team would competitively position Lumakras, a treatment for adult patients with advanced or metastatic non-small cell lung cancer. Their strategy involved analysing the clinical landscape, identifying therapeutic challenges, and outlining a plan to create a patient-centric model, where clinical data would be collected via an app by both doctors and patients. The team pitched that coupling the Lumakras treatment with a digital app to track patient progress would allow doctors and researchers to harness this data pipeline and provide superior care compared to other drugs.
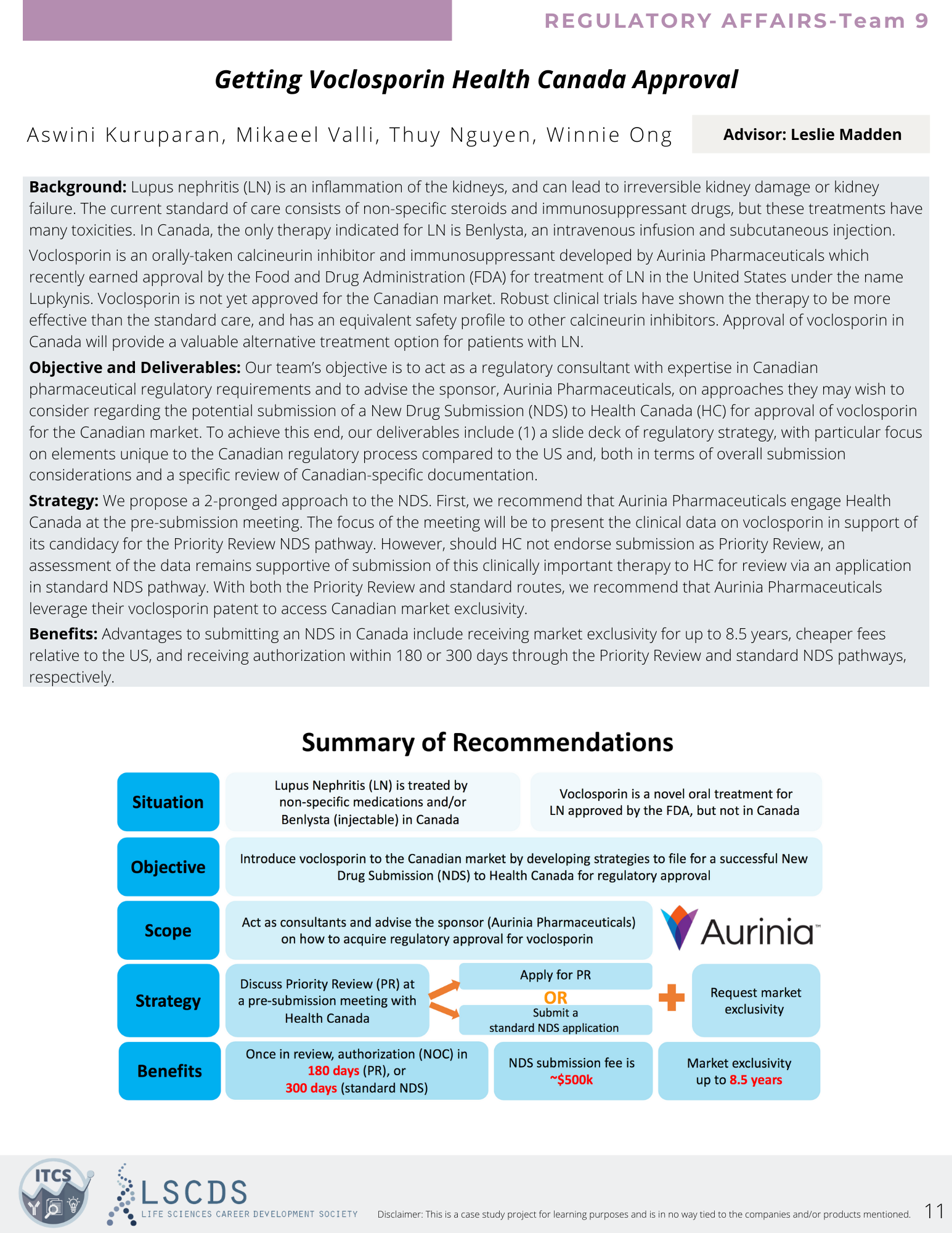
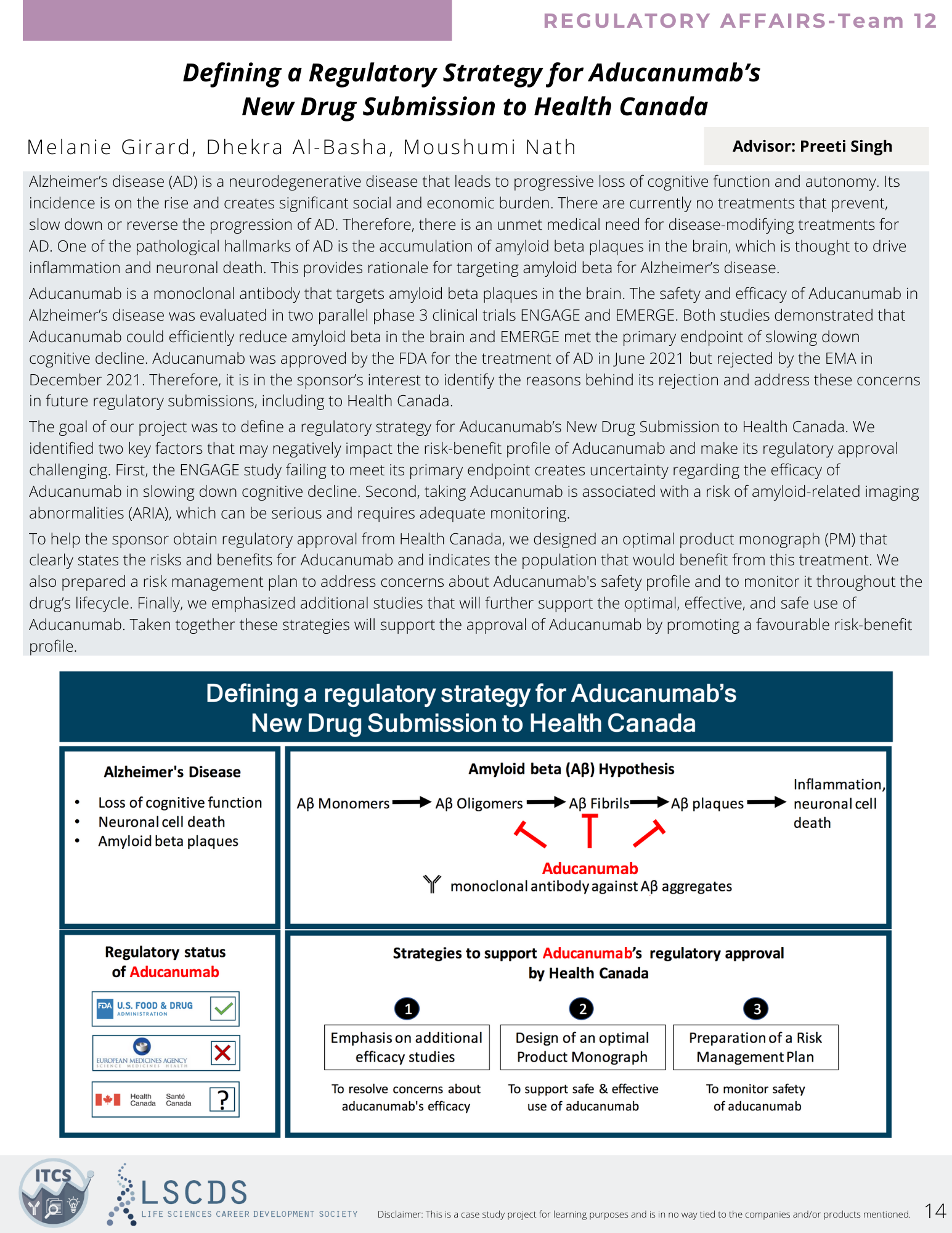
Moving to the regulatory affairs room, I attended an excellent presentation that outlined a strategy to obtain Health Canada’s approval of the only drug found to be effective against Alzheimer’s to date. The team described that the drug, Aducanumab, works to target and remove amyloid-β, the main component of amyloid plaques found in the brains of Alzheimer’s patients. The drug was shown to be successful in one out of three phase III clinical trials, meaning it lacked the necessary 2/3 successes required for approval in Canada. However, the team argued that it being the only effective treatment thus far meant it should be made available to patients if the side effects were understood by doctors and patients. They proposed several strategies to support the approval of the drug from choosing the right regulatory pathway to mitigating risks that could hinder the process.
After attending the presentations, trainees and advisors moved to Hart House, where refreshments were served and everyone had a chance to discuss their experiences and network with industry professionals. I spoke to trainees from the business and market access team who shared that their projects had helped them gain an understanding of the creative marketing strategies that underpin the successful distribution of life-saving drugs. From speaking to trainees in the regulatory affairs team, I learned of the complex process that occurs between discovering a drug in the lab and delivering it to patients. Many students explained that this experience garnered them a better understanding of what happens between the bench and bedside, and has motivated some to leverage their academic research expertise to support these processes. Overall, students believe that participating in the ITCS allowed them to make career-progressing connections and gain insight into what to pursue post-graduation.
The ITCS is one of LSCDS’s flagship events! 44 trainees and 12 advisors participated this year. Approximately, 55% of trainees that participated in ITCS between 2016-2020 were employed in an industry position after graduation. Learn more about the program here and follow LSCDS on Twitter for updates. If you are interested in an industry career, be sure to check out ITCS next year! A big shout out to our trainees, advisors and ITCS executive team, the 2022 Project Report is presented below and the full pdf version is linked here.











Trainees and advisors at the networking event in the Hart House Debates room
The ITCS executive team: (Left to right) Bei Yan, Marie-Eve Di Raddo, Bushra Yusuf, Dorottya Harangi.
ITCS participants being introduced to the Networking session


